Jena. Every living thing is colonized by microbes – fungi, bacteria, archaea and viruses. Together, these make up the microbiome. The human microbiome is composed of trillions of microorganisms that colonize the skin and mucous membranes, forming a metabolically active defense layer against pathogens that is essential for individual health. The composition of the microbiome is unique to each person, and it changes depending on external influences such as diet, infections or contact with other people. Recent findings have shown that the human microbiome also undergoes important changes during the aging process. The ecosystem formed by the human host and its microbes can shift out of balance, just like other ecosystems, causing disease and increasing the risk of death.
The balance of the human-microbe ecosystem is at risk in old age
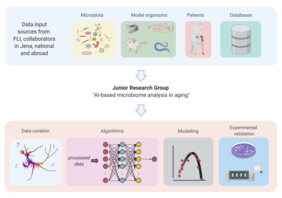
“When the microbiome in the gut loses diversity (i.e. has fewer different types of microorganisms) and becomes dominated by only a few microbial species, pathogens typically join the mix, threatening the host’s health. Infections and inflammatory processes are more likely to occur, triggering a cascade of events that negatively impact the function of several organs, such as the liver and the brain. Such events are more likely to occur in older people,” says Prof. Dario R. Valenzano, who heads the research group “Evolutionary Biology / Microbiome-Host Interactions in Aging” at the Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) in Jena. Together with his colleague at FLI, bioinformatician Prof. Steve Hoffmann, the evolutionary biologist wants to break new ground in understanding how this breakdown in the human-microbe ecosystem leads to disease. How do the trillions of microbes relate to each other and to the body’s cells? To this end, the FLI is establishing a new junior research group in 2022: “AI-based microbiome analysis in aging.” In line with its focus on artificial intelligence, the Carl Zeiss Foundation is funding the project with 2.5 million euros over five years.
New methods for disease prediction
The new research group will develop and apply machine learning and artificial intelligence (AI) methods to gain new insights from biological data. The aim is to use a combination of different methods of data analysis to develop models that allow conclusions to be drawn about the state of disease or health based on the composition of the microbiome.
“In the future, such methods could be increasingly used in the diagnosis of colorectal cancer or inflammatory bowel diseases,” says Steve Hoffmann, who heads the research area “Computational and Systems Biology of Aging” at the FLI. “AI methods can also help us analyze changes in the composition and function of microbiome components over time. This temporal dimension is of course the most important to us; after all, we only age over time,” Hoffmann points out.
The data to “train” such AI-based prediction models have been generated by scientists at the FLI for decades. These include, for example, long-term data series on the transcriptome (the totality of genes transcribed in a cell) during aging, as well as data on the influence of the microbiome on epigenetic changes and data on the composition of fecal samples taken from fish and mice throughout their lifespan.
More insights through better analysis of research data
“Incorporating machine learning methods into microbiome research and aging research provides a tremendous increase in the knowledge that can be gained from complex data collections,” notes Prof. Alfred Nordheim, Scientific Director of the FLI. “We are very grateful to the Carl Zeiss Foundation for supporting this important development at FLI.”
Contact
Kristina Vaillant
Communications Manager
Phone: 03641-65-6373
Email: presse@~@leibniz-fli.de