Kirstein Research Group
Biochemistry of Aging
The long-term health is inextricably linked to protein quality control. An imbalance in protein homeostasis (proteostasis) can result in severe molecular damage to the cell, leading to tissue pathology and enhanced susceptibility to diseases such as metabolic diseases and neurodegeneration, but also cancer and immunodeficiency. Our group aims to uncover the complex mechanisms of the proteostasis network with aging, strategies to cope with protein aggregates and its associated pathologies.
Research focus
We aim to advance our understanding of the mechanisms that maintain a functional proteome during the lifespan of an organism. For that, we use an animal model that has a long-standing history as an excellent aging model, the nematode Caenorhabditis elegans.
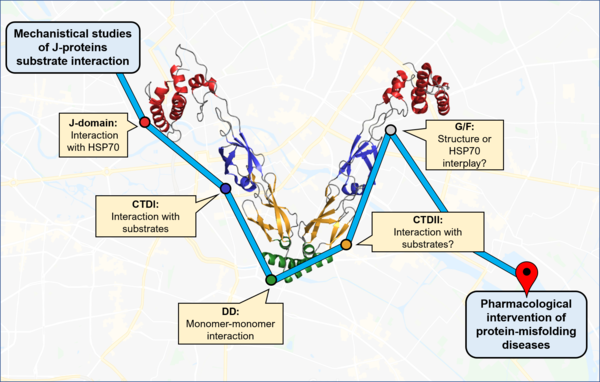
Our knowledge of molecular chaperones and proteolytic machines, how they work, how they recognize a substrate and contribute to protein folding, are almost entirely based on in vitro or ex vivo data. And indeed, our lab uses biochemical and biophysical methods and work with purified individual human molecular chaperones.
We reconstitute functional chaperone complexes and amyloid proteins such as Huntingtin, tau and Ab42in vitro to gain mechanistic insights how molecular chaperones can suppress and reverse aggregation of amyloid proteins.
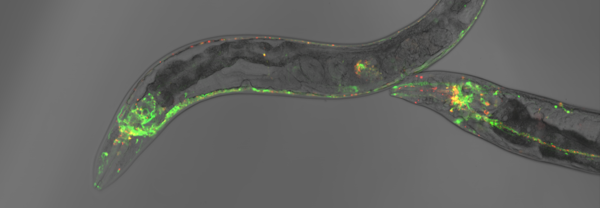
However, we use in addition C. elegans to bridge these mechanistic analyses with physiological studies to monitor protein misfolding and aggregation in a living and aging animal. Specifically, we employ novel proteostasis sensors and advanced imaging techniques to identify key chaperones and proteolytic complexes maintaining proteostasis with the progression of aging and in neurodegenerative disease models for Huntington’s disease and Alzheimer’s disease.
Contact

Janine Kirstein
Group Leader
+49 3641 65-6415
janine.kirstein@~@leibniz-fli.de
Eileen Stöckl
Assistance
+49 3641 65-6815
eileen.stoeckl@~@leibniz-fli.de