GRP Measures at FLI:
An ongoing optimization process
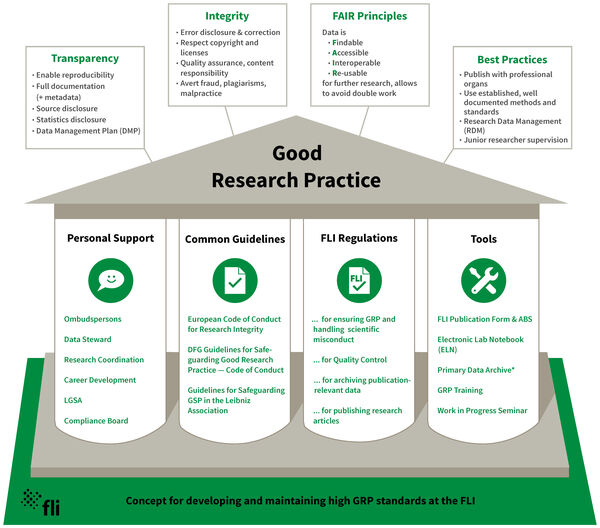
Based on the DFG memorandum on Safeguarding Good Scientific Practice (GSP) 1998, at the Institute of Molecular Biotechnology (IMB), the predecessor institute of the FLI, an ombudsman was appointed and Good Research Practice (GRP, formerly known as GSP) rules tailored for the institute were adopted. Since then, many further measures have been implemented to ensure GRP at FLI. Education and training in the area of GRP compliance are a dynamic processes. This is why improvements, adjustments, and new ideas concerning the framework for GRP at FLI are constantly discussed. With the development of this GRP Concept, further improvements of GRP measures at FLI can be developed and ascertained also in the future.
GRP Measures
From 1999 to 2011, Dr. Jürgen Sühnel acted as the ombudsperson of FLI, Dr. Matthias Görlach from 2011 to 2015, and Prof. Dr. Frank Große from January 2016. Due to illness, Prof. Große was deputized from May 2016 by Dr. Görlach, who held the position from December 1, 2017 until February 28, 2021. His deputy was Prof. Christoph Englert.
Currently, Prof. Christoph Englert and Dr. Susann Groth are the ombudspersons at FLI.
The ombudspersons have conducted over the years many interviews with staff members concerning tasks and potentials of the ombudswork. Moreover, it’s the ombudsperson’s duty to inform all FLI-scientists about current national and international news and developments with regard to GSP. For more detailed information on the duties of an ombudsperson, see here.
Since 2004, a well-established weekly institute seminar has taken place, in which two scientists present their latest findings to the whole institute. The data presented are scrutinized, controls are assessed and suggestions are brought forward.
The Leibniz Graduate School on Aging (LGSA), established in 2005, is a key element for safeguarding GSP at FLI. PhD students are selected and admitted following a standard procedure with an interview by a committee consisting of three group leaders. The mentoring of the PhD student is primarily the responsibility of the group leader who sets the topic. A thesis committee consisting of the mentoring and two further group leaders, one of whom should be external, is set up for every PhD student. The thesis committee meets at least annually, monitors and assesses the progress of the thesis and is available to give consultation to the PhD student (LGSA rules6). Since 2010, the LGSA has organized training courses for all PhD students, which have been mandatory since 2013, on keeping lab records, presentation and GSP.
For more information, visit the website of LGSA.
Since 2007, the FLI rules concerning GRP have been handed out when new staff are employed and they are integral part of the contract of employment since 2011.
Since 2010, GSP courses have been provided annually at FLI by external trainers. To date, a total of 80 PhD students and 11 postdocs have attended these courses. The courses are organized by employees of the Institut für systemische Medizin- und Organisationsethik (Institute of Systemic Medical and Organisational Ethics) in Berlin.
In 2017, the obligatory attendance of GSP courses was extended to group leaders and postdocs. All group leaders of the FLI have been trained in a GSP workshop on 22./23.1.2018 by external experts on the subject. Newly recruited group leaders and postdocs have to attend an appropriate course at the earliest possible date. Long-time group leaders and postdocs have to renew this qualification after five years. The attendance has been documented in the personnel file and is now part of the duties and responsibilities assigned in writing to group leaders.
The FLI has procured an Electronic Lab Notebook (ELN) system, to support the required comprehensive documentation of scientific work at the institute. Beginning in August 2018, the ELN system "RSpace" (ResearchSpace Ltd.) was implemented. Training of all scientific personnel, including the technical assistants, has taken place at the end of 2018. Following a phase-in practice period, RSpace has been introduced as mandatory documentation tool for all scientific staff members as of July 1st 2019.
As a further and complementary measure to an ELN, a task force has been set up to develop and implement a concept for primary data management. The aim is to transfer primary data to a read-only archive to simplify the systematic documentation and safeguarding of results.
A "Guideline Quality Control" for experimental work in the laboratories and facilities has been developed. It aims at safeguarding the reliable collection of data, their reproducibility, traceability, statistical evaluation, and archiving according to GSP rules.
Guideline Quality Control [pdf, internal only]
The archiving of publication-relevant data was previously organized individually by the research groups. The implementation of a central archiving system is in progress. Currently, the publication-relevant data for central archiving are provided electronically. Please see separate "Rules for Archiving Publication-Relevant Data" for details. The FLI “Rules for Publishing Research Articles” and the “Form for Publications” were amended according to the new policies.
Rules for Archiving Publication-Relevant Data [pdf, internal only]
Rules for Publishing Research Articles [pdf, internal only]
Form for Publications [pdf or MS word, internal only]
The institute is employing a computer-based check of publications for data integrity by an external company. The aim is to check manuscripts independently by electronic means for image manipulations, plagiarism and soundness of statistical data. To check for soundness of statistical data, the archived original data will be used. This process is now also represented in the “Form for Publications” and the FLI “Rules for Publishing Research Articles”.
Rules for Publishing Research Articles [pdf, internal only]
Form for Publications [pdf or MS word, internal only]
How To: Archiving and computer-based checking of publications / theses [pdf, internal only]
A scientific FLI member of staff who has previously checked dissertations regarding language was appointed to review these also for compliance with GSP rules. For this purpose a guideline has been established in January 2018.
Downloads & Additional Information
Regulations
For PhD candidates: LGSA Training Guidelines and Rules for PhD-thesis submission [pdf, internal only]
Forms
Form for FLI publications [pdf, internal only]
FLI Dissertation Submission Form (pdf)
Documentation
FTP Server (under development)
- Qualitätszirkel Promotion: new edition of "Shaping a Doctorate Together" for Doctoral Candidates [pdf] and Supervisors [pdf]
- Hochschulrektorenkonferenz: Gute wissenschaftliche Praxis an deutschen Hochschulen [web link]
- Hochschulverband: Resolution [web link] des 50. Hochschulverbandstages 2000 "Selbstkontrolle der Wissenschaft und wissenschaftliches Fehlverhalten"
- The Seven Sins in Academic Behavior in the Natural Sciences [pdf, limited access] by Wilfred F. van Gunsteren (ETH Zürich; Angew. Chem. Intl. Ed. 2013, 52, 118-122)
- On Being A Scientist: Responsible Conduct in Research [web link] (Committee on Science, Engineering and Public Policy, National Academy of Sciences, National Academy of Engineering, Institute of Medicine, National Academy Press, Washington, D.C. 1995)
- UHHS: Online Research Ethics Course [web link]
- Wikipedia Article on Scientific Misconduct [web link]
Predatory journals and questionable conferences:
- Guide to Predatory Publishing by the Leibniz Association [pdf]
Helpful web sites on the topics can be found for journals here and for conferences here.
Relevant Downloads
Additional Downloads
Code for Good Research Practice in the Leibniz Association
Guidelines for Good Scientific Practice in the Leibniz Association
Recommendations GWP Co-Authorship of executive board of the Leibniz Association
DFG Guidelines for Safeguarding Good Research Practice - Code of Conduct
European Code of Conduct for Research Integrity by ALLEA - All European Academies
World Economics Forum Code of Ethics [pdf]
ENRIO Statement COVID-19 [pdf]