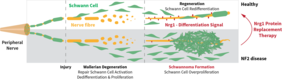
Jena. A schwannoma is a benign tumor of the peripheral nervous system. It usually results from an abnormal overgrowth of Schwann cells, a cell type covering nerve fibers and supporting them in their function. Following nerve damage, Schwann cells divide to support regeneration in sufficient numbers. However, if cell division is not limited in time, over-proliferation of Schwann cells occurs and causes schwannoma formation. These progressively growing tumors compress nerve cells, impairing nerve function and causing symptoms such as paralysis or pain. Schwannomas often appear in the context of the hereditary disease neurofibromatosis type 2 (NF2), which causes uncontrolled growth of nervous and connective tissues, particularly surrounding the 8th cranial nerve. Consequently, loss of hearing and balance are frequent symptoms of NF2.
Prof. Dr. Helen Morrison and her research group “Nerve Regeneration” at the Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) in Jena, Germany, is studying the role of Schwann cells in the peripheral nervous system. The team was able to identify a molecule that inhibits schwannoma growth. The protein Neuregulin 1 (Nrg1) occurs naturally in the body and induces differentiation of Schwann cells, including termination of cell division following nerve regeneration. However, in the case of the hereditary disease NF2 and likely other schwannomas, Nrg1 is strongly downregulated. Without this stop signal, Schwann cells fail to differentiate, continue to divide and form schwannomas. Therapy options are very limited and focus on surgical removal, often causing additional nerve damage and entailing a high recurrence rate. Novel approaches are urgently needed to improve tumor therapy and patient’s quality of life.
Prof. Dr. Morrison's group has already shown that Nrg1 inhibits schwannoma growth and improves nerve function in a mouse model. Her current project aims to develop this approach further towards clinical application. Previous studies on heart diseases have classified Nrg1 as safe for use in humans. Now, the research group has successfully obtained funding to conduct a multi-centric, preclinical study. Starting from August 2020, the German Federal Ministry of Education and Research (BMBF) will fund this study for 3 years with a total sum of 1.45 million euro, of which approximately 732,000 euro will go to FLI.
Animal study according to standards of clinical patient studies
We are very pleased about this support and see this as encouragement of our research," says Prof. Morrison, who has many years of experience in research on the hereditary disease NF2. "Together with our partners we hope to contribute to the development of a drug to help people affected by this disease." Headed by Prof. Dr. Morrison of the FLI, the research team also includes the partner laboratories of Dr. Robert Fledrich and Dr. Ruth Stassart of the University Hospital Leipzig and Prof. Dr. Reinhard Bauer at the Institute of Molecular Cell Biology of Jena University Hospital. The teams‘ research approach is unprecedented: Following standards of clinical patient trials, three centers will conduct parallel, randomized and blinded experiments following a preregistered study protocol. Study design as well as statistical data analysis is conducted independently by the team of Prof. Dr. André Scherag of the Institute for Medical Statistics, Computer and Data Sciences of Jena University Hospital. Collectively, these measures strongly increase the studies’ validity and significance.
The research network will be further supported by a funded membership of the TMF (Technologie- und Methodenplattform für die vernetzte medizinische Forschung e.V.), that will enable a broad exchange with experts on diverse methods crucial to networked medical research (e.g. research data management). Additionally, all projects of the current BMBF funding line will be counseled by the partner project “DECIDE” located at the Charité in Berlin. “We are looking forward to the interaction with all these different experts to apply highest standards to our preclinical study,” concludes Prof. Dr. Morrison.
The new BMBF funding line for preclinical studies supports innovative research projects aiming to expedite translation of important research findings from basic research to clinical application. This aim is shared by the SPARK@FLI program, which supported the preceding work leading up to the current preclinical study. Dr. Sonja Schätzlein, head of SPARK@FLI, sees the current funding as a success: "I am pleased to see that SPARK has contributed to advancing the project to this important step towards an application-oriented therapy by providing financial support, advice and arranging mentoring partners from industry.”
The project is funded by the Federal Ministry of Education and Research (BMBF), funding code 01KC2003A.
Contact
Magdalena Voll
Press and Public Relations
Phone: 03641 656378, email: presse@~@leibniz-fli.de