Jena/Senftenberg. Rhabdomyosarcoma, a malignant tumor, is one of the most common soft tissue tumors occurring during childhood. The tumors are associated with skeletal muscle and can emerge anywhere in the body. Consequently, the clinical presentation is very diverse and complete removal of the tumors is often difficult and sometimes nearly impossible due to their location. This is why intensive research is being conducted into alternative treatment approaches.
A team led by Prof. Julia von Maltzahn, a former research group leader at the Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) and now professor of Stem Cell Biology of Aging at the Brandenburg University of Technology Cottbus-Senftenberg (BTU), has discovered a new treatment approach for rhabdomyosarcoma that could be used as a complemental treatment supporting traditional chemotherapy and radiation therapy. By targeting tumor cells and transforming them into muscle cells, her team was able to stop the growth of the cells and, thereby, tumor growth. The research results have now been published in the renowned journal Molecular Therapy.
Increased TRPS1 levels in soft tissue tumors
Rhabdomyosarcomas can occur as two subtypes, the alveolar and the embryonal subtype. In the embryonal subtype, the molecular causes of tumor progression are largely unknown so far or very diverse, making it difficult to develop therapeutic approaches. Current knowledge suggests that tumors arise from muscle progenitor cells.
“Through our studies on human embryonal rhabdomyosarcoma tumor cells, we found that the levels of the transcriptional repressor TRPS1, a protein that can bind to DNA and thus repress gene expression, are consistently elevated,” Prof. von Maltzahn explained. In skeletal muscle tissue these increased TRPS1 levels then lead to impaired differentiation of stem cells and in embryonic rhabdomyosarcoma cells to increased cell growth, which consequently promotes the tumor growth.
Influence of TRPS1 on stem cell differentiation
“During normal development of skeletal muscle, TRPS1 levels must be downregulated in cells at the onset of differentiation, since only then a proper development and growth of muscle can be achieved,” Dr. Sören S. Hüttner adds, who conducted the studies during his PhD thesis at the FLI. However, the question remains what is the exact role of TRPS1 in myogenic differentiation?
The researchers found that aberrantly high levels of TRPS1 in normal muscle cells severely impaired muscle formation (myogenesis), similar to what can be observed in tumors. “Thus, elevated TRPS1 levels appear to be one of the main inhibitors of terminal myogenic differentiation, interfering with skeletal muscle formation and development,” Prof. von Maltzahn reported. “This made us come up with the idea that decreasing TRPS1 levels in tumor cells might reduce tumor growth.”
Reprogramming – Tumor cells become muscles cells again
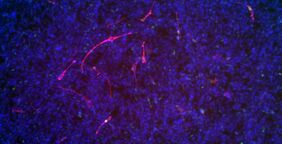
Through collaboration with Dr. Björn von Eyss from the FLI and Prof. Ursula Anderer from the BTU and by the use of various techniques (e.g. shRNAs, Crispr/Cas9), the researchers actually succeeded in demonstrating – in cell culture and in a mouse model – that the targeted reduction of TRPS1 levels in tumor cells can drive differentiation: the tumor cells become muscle cells. At locations where a tumor was located beforehand, muscle cells start to develop, a cell type which cannot divide anymore.
New treatment approach for soft tissue tumors
“Based on these promising results, we believe that we found an effective, new therapeutic approach for the treatment of embryonal rhabdomyosarcomas, which could be used in the future – in addition to traditional chemotherapy and radiotherapy – to stop the growth and spreading of the tumors,” Dr. Hüttner explains, emphasizing the relevance of the results.
The research findings are expected to have far-reaching implications for cancer research, particularly for the study of soft tissue tumors in children and the development of new strategies for the treatment of rhabdomyosarcoma. All indications are suggesting that TRPS1 is a promising therapeutic target with high potential to transform tumor cells into muscle cells, a cell type which cannot undergo cell divisions anymore, thus helping to stop tumor growth.
Publication
A dysfunctional miR-1-TRPS1-MYOG axis drives ERMS by suppressing terminal myogenic differentiation. Hüttner SS, Henze H, Elster D, Koch P, Anderer U, von Eyss B, von Maltzahn J. Mol Ther. 2023, S1525-0016(23)00383-0. doi: 10.1016/j.ymthe.2023.07.003.
https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(23)00383-0
The project “Driving rhabdomyosarcoma into differentiation” was funded as part of SPARK-FLI from 2020–2021. The internal technology transfer program SPARK-FLI helps to translate fundamental scientific findings from biomedical research into diagnostics or medical applications and ensures that government-funded research contributes to improving the health of our society. This targeted funding not only increases the chances of success of the respective research project, but also reduces the costs as well as the time required to achieve the goal.
Contact
FLI:
Dr. Kerstin Wagner
Press and Public Relations
Phone: 03641-656378, email: presse@~@leibniz-fli.de
BTU:
Ilka Seer
Press Officer, Head of Corporate Identity Department
Phone: 0355-693612, email: ilka.seer@~@b-tu.de