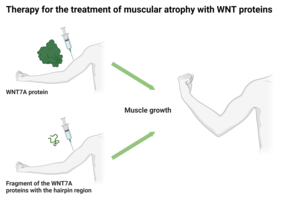
Jena/Cottbus-Senftenberg. WNT signaling is crucial for the development of organisms and the maintenance of tissue homeostasis, since the WNT messengers are involved in a variety of cellular functions. In skeletal muscle, for example, they can increase muscle mass and improve regeneration. This is an important entry point for therapies to treat diseases associated with reduced muscle mass, such as cachexia. Despite this promising potential, until now it has been difficult to use the potential of these molecules in practice: the messenger proteins, which consist of more than 400 amino acids, are very large and “sticky” – meaning, they show poor dispersal in the tissue, which makes medical application difficult. Furthermore, little is known about the functional relevance of the different structural areas in the individual WNT proteins.
The research group “Stem Cells of Skeletal Muscle” led by Prof. Julia von Maltzahn from the Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) in Jena and the Faculty of Health Sciences at the BTU Cottbus-Senftenberg in Senftenberg, has now demonstrated in a recent study, that even a small component of the messenger protein WNT7A is sufficient to activate the same signaling pathways in muscle cells which can be activated by the complete protein. The study was recently published in the Computational and Structural Biotechnology Journal (Open Access).
Which areas are important for WNT signal transmission?
“So far, very little is known about the importance and functional relevance of the different areas in WNT proteins, especially about the binding sites to the receptors in recipient cells,” reports Prof. Julia von Maltzahn. “Therefore, we specifically investigated the C-terminus of WNT7A, as this region contains both a hairpin and a linker region and is shows full functionality.”
Hairpin region is sufficient to promote muscle growth
The researchers found that treatment with small protein fragments, the C-terminal WNT7A variants containing only the hairpin region, is sufficient to trigger the same cellular signaling pathways in muscle cells that are activated by the complete WNT7A protein. The extracellular messenger WNT7A influences the signaling pathway for muscle growth and the renewal of muscle stem cells. This stimulates the growth of muscle fibers and promotes proliferation and distribution of muscle stem cells in skeletal muscle, for example after injury.
Will it soon be possible to treat muscular atrophy with tiny messenger fragments?
“Our results demonstrated, that tiny messenger fragments, consisting of only the hairpin region, make the development of a WNT7A-based therapy for various muscle wasting conditions, such as cancer cachexia, more feasible,” explains Dr. Manuel Schmidt, postdoc in the research group and lead author of the publication.
In previous studies, the Jena scientists had already shown that WNT7A increases muscle mass and the division of muscle stem cells in mice suffering from cachexia, a muscle-wasting syndrome that affects a large proportion of cancer patients. The WNT proteins were subsequently targeted by researchers as promising candidates for therapeutic interventions to address muscle-wasting diseases such as muscular dystrophy and cancer cachexia, but the molecules’ considerable size and poor manageability prevent their use as a therapeutic agent.
A wide range of possible applications
The shorter variant of the protein could now solve this problem. Thanks to its small size, the extracellular messenger WNT7A seems to be better suited for therapeutic use. Possible applications include supportive therapies for the loss of muscle mass due to cancer or as a stimulus for muscle regeneration after surgery. This is due to the fact that WNT7A promotes an increase of stem cells in the muscle and thus supports muscle regeneration after injuries or surgeries.
The work was funded by the German Research Foundation (DFG) and the German Cancer Aid Foundation (Deutsche Krebshilfe) and supported by the SPARK-FLI technology transfer program at the institute. Dr. Sonja Schätzlein, head of SPARK-FLI, considers the research results an initial success: “I am very pleased that through our financial support, advice, and provision of mentoring partners from industry, SPARK-FLI was able to help advance this project an important step towards a medical therapy. I eagerly look forward to seeing further results.”
Publication
Schmidt M, Poser C, Janster C, von Maltzahn J. The hairpin region of WNT7A is sufficient for binding to the Frizzled7 receptor and to elicit signaling in myogenic cells. Computational and Structural Biotechnology Journal. 2022, 20, 6348-6359. DOI: 10.1016/j.csbj.2022.10.047.
https://www.sciencedirect.com/science/article/pii/S2001037022004949
Information on SPARK-FLI
The “Counteracting Cancer Cachexia” project was supported from 2018–2020 by SPARK-FLI. The internal technology transfer program SPARK-FLI helps to transfer fundamental scientific findings from biomedical research into diagnostics or medical applications and ensures that government-funded research contributes to the improved health of our society. This targeted funding not only increases the chances of success of the respective research project, but also reduces the costs as well as the time required to achieve its aims.
Contact
Dr. Kerstin Wagner
Press and Public Relations
Phone: 03641-656378, email: presse@~@leibniz-fli.de