Subarea 1: Stem Cell Aging
The individual research groups within Subarea 1 investigate the causes and consequences of stem cell aging. The research work spans from basic model organisms over genetic mouse models up to humanized mouse models engrafted with human stem cells.
According to the FLI, with the closure of two groups since 2016 the representation of invertebrate models of stem cell research was reduced in Subarea 1. The institute presumes that the recruitment of new groups should fill this gap.
The research is defined by four focus areas:
- Cell-intrinsic mechanisms limiting the function of aging stem and progenitor cells,
- Aging-associated alterations of stem cell niches and the systemic environment,
- Mechanisms of clonal selection and epigenetic drifts in stem cell aging, and
- Microbiota- and metabolism-induced impairments in stem cell function during aging (in context of the new focus area Microbiota and Aging currently being built up within Subarea 2).
Research focus of Subarea 1.
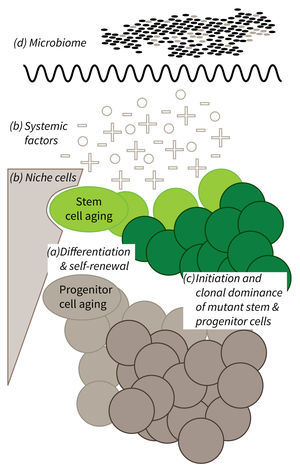
a) It is currently not well understood what mechanisms impair cellular functions in aging. b) The relative contribution of niche cells and systemic acting factors on stem cell aging have yet to be determined in different tissues. c) Clonal expansion of mutant cells associates with disease development in aging humans. Mechanistically, the process remains poorly understood. Changes in color intensity depict clonal dominance originating from stem (green) or progenitor cells (gray). d) Emerging evidences indicate that aging associated alter ations in microbiota influence stem cell function and vice versa.
Publications
(since 2016)
2025
- Embryonic macrophages orchestrate niche cell homeostasis for the establishment of the definitive hematopoietic stem cell pool.
Perçin** G, Riege K, Fröbel J, Metz J, Culemann S, Lesche M, Reinhardt S, Höfer T, Hoffmann S, Waskow** C
Nat Commun 2025, 16(1), 4428 ** co-corresponding authors - Malignant JAK-signaling: at the interface of inflammation and malignant transformation.
Perner F, Pahl HL, Zeiser R, Heidel FH
Leukemia 2025, 39(5), 1011-30 - Mesenchymal Stromal Cells regulate human Hematopoietic Stem Cell survival, engraftment and regeneration via PGE2/cAMP signaling pathway
Sai Naga Anurag Muddineni S, Katz Even C, Zipin-Roitman A, Rasoulouniriana D, Singha Roy D, Aborgies R, Beider K, Raz Y, Solomon N, Hershkovitz G, Shulman Y, Chen R, Weizman E, Waskow C, Nagler A, Milyavsky M
bioRxiv 2025, https://doi.org/10.1101/2023.11. - Anti-CD19 CAR-T cell therapy for acquired hemophilia A.
Schultze-Florey CR, Thol FR, Aleksandrova K, Stoyanov K, Gutierrez Jauregui R, Arseniev L, Leise J, Klöß S, Heidel** FH, Tiede** A
Leukemia 2025, 39(4), 980-2 ** co-corresponding authors - Enhancer Hijacking Discovery in Acute Myeloid Leukemia by Pyjacker Identifies MNX1 Activation via Deletion 7q.
Sollier E, Riedel A, Toprak UH, Wierzbinska JA, Weichenhan D, Schmid JP, Hakobyan M, Touzart A, Jahn E, Vick B, Brown-Burke F, Kelly K, Kelekçi S, Pejkovska A, Goyal A, Bähr M, Breuer K, Chen MJM, Llamazares-Prada M, Hartmann M, Schönung M, Correia N, Trumpp A, Abdullah Y, Klingmüller U, Mughal SS, Brors B, Westermann F, Ulrich E, Autry RJ, Schlesner M, Vosberg S, Herold T, Greif PA, Pfeifer D, Lübbert M, Fischer T, Heidel FH, Gebhard C, Walter W, Haferlach T, Eisfeld AK, Mrózek K, Nicolet D, Bullinger L, Smeenk L, Erpelinck-Verschueren C, Mulet-Lazaro R, Delwel R, Ernst A, Scherer M, Lutsik P, Jeremias I, Döhner K, Döhner H, Lipka DB, Plass C
Blood Cancer Discov 2025, 6(4), 343-63
2024
- Imetelstat-mediated alterations in fatty acid metabolism to induce ferroptosis as a therapeutic strategy for acute myeloid leukemia.
Bruedigam C, Porter AH, Song A, Vroeg In de Wei G, Stoll T, Straube J, Cooper L, Cheng G, Kahl VFS, Sobinoff AP, Ling VY, Jebaraj BMC, Janardhanan Y, Haldar R, Bray LJ, Bullinger L, Heidel FH, Kennedy GA, Hill MM, Pickett HA, Abdel-Wahab O, Hartel G, Lane SW
Nat Cancer 2024, 5(1), 47-65 - Dietary restriction interventions: lifespan benefits need resilience and are limited by immune compromise and genetics.
Chen Y, Malik A, Rudolph KL
Signal Transduct Target Ther 2024, 9(1), 335 - Dominant immune tolerance in the intestinal tract imposed by RelB-dependent migratory dendritic cells regulates protective type 2 immunity.
Geiselhöringer AL, Kolland D, Patt AJ, Hammann L, Köhler A, Kreft L, Wichmann N, Hils M, Ruedl C, Riemann M, Biedermann T, Anz D, Diefenbach A, Voehringer D, Schmidt-Weber CB, Straub T, Pasztoi M, Ohnmacht C
Nat Commun 2024, 15(1), 9143 - Denervation alters the secretome of myofibers and thereby affects muscle stem cell lineage progression and functionality.
Henze H, Hüttner SS, Koch P, Schüler SC, Groth M, von Eyss B, von Maltzahn J
NPJ Regen Med 2024, 9(1), 10 - The oncogenic lncRNA MIR503HG suppresses cellular senescence counteracting supraphysiological androgen treatment in prostate cancer.
Kallenbach J, Rasa M, Heidari Horestani M, Atri Roozbahani G, Schindler K, Baniahmad A
J Exp Clin Cancer Res 2024, 43(1), 321