Subarea 1: Stem Cell Aging
The individual research groups within Subarea 1 investigate the causes and consequences of stem cell aging. The research work spans from basic model organisms over genetic mouse models up to humanized mouse models engrafted with human stem cells.
According to the FLI, with the closure of two groups since 2016 the representation of invertebrate models of stem cell research was reduced in Subarea 1. The institute presumes that the recruitment of new groups should fill this gap.
The research is defined by four focus areas:
- Cell-intrinsic mechanisms limiting the function of aging stem and progenitor cells,
- Aging-associated alterations of stem cell niches and the systemic environment,
- Mechanisms of clonal selection and epigenetic drifts in stem cell aging, and
- Microbiota- and metabolism-induced impairments in stem cell function during aging (in context of the new focus area Microbiota and Aging currently being built up within Subarea 2).
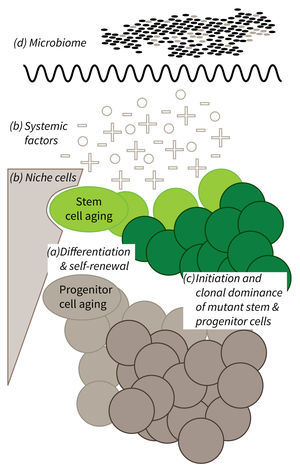
Research focus of Subarea 1.
a) It is currently not well understood what mechanisms impair cellular functions in aging. b) The relative contribution of niche cells and systemic acting factors on stem cell aging have yet to be determined in different tissues. c) Clonal expansion of mutant cells associates with disease development in aging humans. Mechanistically, the process remains poorly understood. Changes in color intensity depict clonal dominance originating from stem (green) or progenitor cells (gray). d) Emerging evidences indicate that aging associated alter ations in microbiota influence stem cell function and vice versa.
Publications
(since 2016)
2021
- Granulocyte colony-stimulating factor acts on lymphoid-biased, short-term hematopoietic stem cells.
Chen Y, Rudolph KL
Haematologica 2021, 106(6), 1516-8 - Enhanced differentiation of functional human T cells in NSGW41 mice with tissue-specific expression of human interleukin-7.
Coppin* E, Sundarasetty* BS, Rahmig S, Blume J, Verheyden NA, Bahlmann F, Ravens S, Schubert U, Schmid J, Ludwig S, Geissler K, Guntinas-Lichius O, von Kaisenberg C, Groten T, Platz A, Naumann R, Ludwig B, Prinz I, Waskow** C, Krueger** A
Leukemia 2021, 35(12), 3561-7 * equal contribution, ** co-senior authors - Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition).
Cossarizza A, Chang HD, Radbruch A, Abrignani S, Addo R, Akdis M, Andrä I, Andreata F, Annunziato F, Arranz E, Bacher P, Bari S, Barnaba V, Barros-Martins J, Baumjohann D, Beccaria CG, Bernardo D, Boardman DA, Borger J, Böttcher C, Brockmann L, Burns M, Busch DH, Cameron G, Cammarata I, Cassotta A, Chang Y, Chirdo FG, Christakou E, Čičin-Šain L, Cook L, Corbett AJ, Cornelis R, Cosmi L, Davey MS, De Biasi S, De Simone G, Del Zotto G, Delacher M, Di Rosa F, Santo JD, Diefenbach A, Dong J, Dörner T, Dress RJ, Dutertre CA, Eckle SBG, Eede P, Evrard M, Falk CS, Feuerer M, Fillatreau S, Fiz-Lopez A, Follo M, Foulds GA, Fröbel J, Gagliani N, Galletti G, Gangaev A, Garbi N, Garrote JA, Geginat J, Gherardin NA, Gibellini L, Ginhoux F, Godfrey DI, Gruarin P, Haftmann C, Hansmann L, Harpur CM, Hayday AC, Heine G, Hernández DC, Herrmann M, Hoelsken O, Huang Q, Huber S, Huber JE, Huehn J, Hundemer M, Hwang WYK, Iannacone M, Ivison SM, Jäck HM, Jani PK, Keller B, Kessler N, Ketelaars S, Knop L, Knopf J, Koay HF, Kobow K, Kriegsmann K, Kristyanto H, Krueger A, Kuehne JF, Kunze-Schumacher H, Kvistborg P, Kwok I, Latorre D, Lenz D, Levings MK, Lino AC, Liotta F, Long HM, Lugli E, MacDonald KN, Maggi L, Maini MK, Mair F, Manta C, Manz RA, Mashreghi MF, Mazzoni A, McCluskey J, Mei HE, Melchers F, Melzer S, Mielenz D, Monin L, Moretta L, Multhoff G, Muñoz LE, Muñoz-Ruiz M, Muscate F, Natalini A, Neumann K, Ng LG, Niedobitek A, Niemz J, Almeida LN, Notarbartolo S, Ostendorf L, Pallett LJ, Patel AA, Percin GI, Peruzzi G, Pinti M, Pockley AG, Pracht K, Prinz I, Pujol-Autonell I, Pulvirenti N, Quatrini L, Quinn KM, Radbruch H, Rhys H, Rodrigo MB, Romagnani C, Saggau C, Sakaguchi S, Sallusto F, Sanderink L, Sandrock I, Schauer C, Scheffold A, Scherer HU, Schiemann M, Schildberg FA, Schober K, Schoen J, Schuh W, Schüler T, Schulz AR, Schulz S, Schulze J, Simonetti S, Singh J, Sitnik KM, Stark R, Starossom S, Stehle C, Szelinski F, Tan L, Tarnok A, Tornack J, Tree TIM, van Beek JJP, van de Veen W, van Gisbergen K, Vasco C, Verheyden NA, von Borstel A, Ward-Hartstonge KA, Warnatz K, Waskow C, Wiedemann A, Wilharm A, Wing J, Wirz O, Wittner J, Yang JHM, Yang J
Eur J Immunol 2021, 51(12), 2708-3145 - Tnfaip2/exoc3-driven lipid metabolism is essential for stem cell differentiation and organ homeostasis.
Deb S, Felix DA, Koch P, Deb MK, Szafranski K, Buder K, Sannai M, Groth M, Kirkpatrick J, Pietsch S, Gollowitzer A, Groß A, Riemenschneider P, Koeberle A, González-Estévez** C, Rudolph** KL
EMBO Rep 2021, 22(1), e49328 ** co-corresponding authors - Molecular Mechanisms of Senescence and Implications for the Treatment of Myeloid Malignancies.
Ernst P, Heidel FH
Cancers (Basel) 2021, 13(4) - Modulation of FLT3-ITD Localization and Targeting of Distinct Downstream Signaling Pathways as Potential Strategies to Overcome FLT3-Inhibitor Resistance.
Fleischmann M, Fischer M, Schnetzke U, Fortner C, Kirkpatrick J, Heidel FH, Hochhaus A, Scholl S
Cells 2021, 10(11), 2992 - GMPPA defects cause a neuromuscular disorder with α-dystroglycan hyperglycosylation.
Franzka P, Henze* H, Jung* MJ, Schüler SC, Mittag S, Biskup K, Liebmann L, Kentache T, Morales J, Martínez B, Katona I, Herrmann T, Huebner AK, Hennings JC, Groth S, Gresing LJ, Horstkorte R, Marquardt T, Weis J, Kaether C, Mutchinick OM, Ori A, Huber O, Blanchard V, von Maltzahn J, Hübner CA
J Clin Invest 2021, 131(9), e139076 * equal contribution - The Hematopoietic Bone Marrow Niche Ecosystem.
Fröbel J, Landspersky T, Percin G, Schreck C, Rahmig S, Ori A, Nowak D, Essers M, Waskow C, Oostendorp RAJ
Front Cell Dev Biol 2021, 9, 705410 - MLL1 is required for maintenance of intestinal stem cells.
Goveas N, Waskow C, Arndt K, Heuberger J, Zhang Q, Alexopoulou D, Dahl A, Birchmeier W, Anastassiadis K, Stewart AF, Kranz A
PLoS Genet 2021, 17(12), e1009250 published during change of institution - Regeneration in starved planarians depends on TRiC/CCT subunits modulating the unfolded protein response.
Gutiérrez-Gutiérrez* Ó, Felix* DA, Salvetti A, Amro EM, Thems A, Pietsch S, Koeberle A, Rudolph KL, González-Estévez C
EMBO Rep 2021, 22(8), e52905 * equal contribution