Subarea 1: Stem Cell Aging
The individual research groups within Subarea 1 investigate the causes and consequences of stem cell aging. The research work spans from basic model organisms over genetic mouse models up to humanized mouse models engrafted with human stem cells.
According to the FLI, with the closure of two groups since 2016 the representation of invertebrate models of stem cell research was reduced in Subarea 1. The institute presumes that the recruitment of new groups should fill this gap.
The research is defined by four focus areas:
- Cell-intrinsic mechanisms limiting the function of aging stem and progenitor cells,
- Aging-associated alterations of stem cell niches and the systemic environment,
- Mechanisms of clonal selection and epigenetic drifts in stem cell aging, and
- Microbiota- and metabolism-induced impairments in stem cell function during aging (in context of the new focus area Microbiota and Aging currently being built up within Subarea 2).
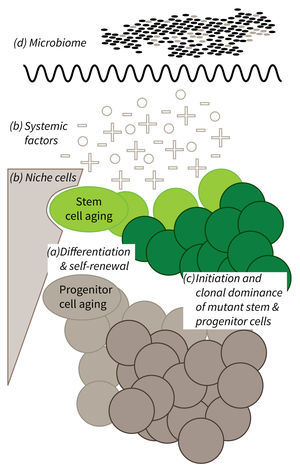
Research focus of Subarea 1.
a) It is currently not well understood what mechanisms impair cellular functions in aging. b) The relative contribution of niche cells and systemic acting factors on stem cell aging have yet to be determined in different tissues. c) Clonal expansion of mutant cells associates with disease development in aging humans. Mechanistically, the process remains poorly understood. Changes in color intensity depict clonal dominance originating from stem (green) or progenitor cells (gray). d) Emerging evidences indicate that aging associated alter ations in microbiota influence stem cell function and vice versa.
Publications
(since 2016)
2019
- Tnfaip2 induces differentiation of pluripotent stem cells and inhibits induction of pluripotency
Deb S
Dissertation 2019, Jena, Germany - It is not all about regeneration: planarians striking power to stand starvation.
Felix DA, Gutiérrez-Gutiérrez Ó, Espada L, Thems A, González-Estévez C
Semin Cell Dev Biol 2019, 87, 169-81 - Hepatic leukemia factor is a novel leukemic stem cell regulator in DNMT3A, NPM1, and FLT3-ITD triple-mutated AML.
Garg S, Reyes-Palomares A, He L, Bergeron A, Lavallée VP, Lemieux S, Gendron P, Rohde C, Xia J, Jagdhane P, Müller-Tidow C, Lipka DB, Imren S, Humphries RK, Waskow C, Vick B, Jeremias I, Richard-Carpentier G, Hébert J, Sauvageau G, Zaugg J, Barabé F, Pabst C
Blood 2019, 134(3), 263-76 - Comparative RNAi Screens in Isogenic Human Stem Cells Reveal SMARCA4 as a Differential Regulator.
Güneş C, Paszkowski-Rogacz M, Rahmig S, Khattak S, Camgöz A, Wermke M, Dahl A, Bornhäuser M, Waskow C, Buchholz F
Stem Cell Reports 2019, 12(5), 1084-98 - Reverse genetic screening identifies essential ribosomal protein genes in hematopoietic stem cells that are dysregulated during aging
Han B
Dissertation 2019, Jena, Germany - Influence of telomere dysfunction and Polq-dependent alternative end-joining on organism aging
Huber NMB
Dissertation 2019, Jena, Germany - Isolation and Culture of Individual Myofibers and Their Adjacent Muscle Stem Cells from Aged and Adult Skeletal Muscle.
Hüttner SS, Ahrens HE, Schmidt M, Henze H, Jung MJ, Schüler SC, von Maltzahn J
Methods Mol Biol 2019, 2045, 25-36 - Downregulation of mTOR Signaling Increases Stem Cell Population Telomere Length during Starvation of Immortal Planarians.
Iglesias M, Felix DA, Gutiérrez-Gutiérrez Ó, De Miguel-Bonet MDM, Sahu S, Fernández-Varas B, Perona R, Aboobaker AA, Flores** I, González-Estévez** C
Stem Cell Reports 2019, 13(2), 405-18 ** co-corresponding authors - Developmental origin, functional maintenance and genetic rescue of osteoclasts.
Jacome-Galarza* CE, Percin* GI, Muller* JT, Mass* E, Lazarov T, Eitler J, Rauner M, Yadav VK, Crozet L, Bohm M, Loyher PL, Karsenty G, Waskow** C, Geissmann** F
Nature 2019, 568(7753), 541-5 * equal contribution, ** co-corresponding authors - The acetyltransferase GCN5 maintains ATRA-resistance in non-APL AML.
Kahl M, Brioli A, Bens M, Perner F, Kresinsky A, Schnetzke U, Hinze A, Sbirkov Y, Stengel S, Simonetti G, Martinelli G, Petrie K, Zelent A, Böhmer FD, Groth M, Ernst T, Heidel FH, Scholl S, Hochhaus A, Schenk T
Leukemia 2019, 33(11), 2628-39