Subarea 1: Stem Cell Aging
The individual research groups within Subarea 1 investigate the causes and consequences of stem cell aging. The research work spans from basic model organisms over genetic mouse models up to humanized mouse models engrafted with human stem cells.
According to the FLI, with the closure of two groups since 2016 the representation of invertebrate models of stem cell research was reduced in Subarea 1. The institute presumes that the recruitment of new groups should fill this gap.
The research is defined by four focus areas:
- Cell-intrinsic mechanisms limiting the function of aging stem and progenitor cells,
- Aging-associated alterations of stem cell niches and the systemic environment,
- Mechanisms of clonal selection and epigenetic drifts in stem cell aging, and
- Microbiota- and metabolism-induced impairments in stem cell function during aging (in context of the new focus area Microbiota and Aging currently being built up within Subarea 2).
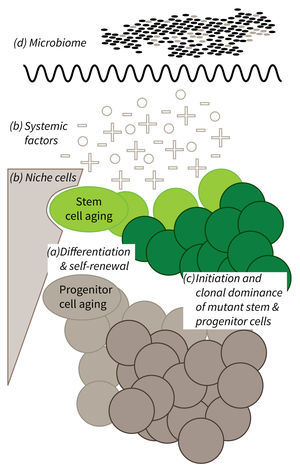
Research focus of Subarea 1.
a) It is currently not well understood what mechanisms impair cellular functions in aging. b) The relative contribution of niche cells and systemic acting factors on stem cell aging have yet to be determined in different tissues. c) Clonal expansion of mutant cells associates with disease development in aging humans. Mechanistically, the process remains poorly understood. Changes in color intensity depict clonal dominance originating from stem (green) or progenitor cells (gray). d) Emerging evidences indicate that aging associated alter ations in microbiota influence stem cell function and vice versa.
Publications
(since 2016)
2022
- Disrupting PTPRJ transmembrane-mediated oligomerization counteracts oncogenic receptor tyrosine kinase FLT3 ITD.
Schwarz M, Rizzo S, Paz WE, Kresinsky A, Thévenin D, Müller JP
Front Oncol 2022, 12, 1017947 - pH-sensitive packaging of cationic particles by an anionic block copolymer shell.
Solomun JI, Martin L, Mapfumo P, Moek E, Amro E, Becker F, Tuempel S, Hoeppener S, Rudolph KL, Traeger A
J Nanobiotechnology 2022, 20(1), 336 - Age-dependent effects of Igf2bp2 on gene regulation, function, and aging of hematopoietic stem cells in mice.
Suo M, Rommelfanger MK, Chen Y, Amro EM, Han B, Chen Z, Szafranski K, Chakkarappan SR, Boehm BO, MacLean AL, Rudolph KL
Blood 2022, 139(17), 2653-65 - PI(18:1/18:1) is a SCD1-derived lipokine that limits stress signaling.
Thürmer M, Gollowitzer A, Pein H, Neukirch K, Gelmez E, Waltl L, Wielsch N, Winkler R, Löser K, Grander J, Hotze M, Harder S, Döding A, Meßner M, Troisi F, Ardelt M, Schlüter H, Pachmayr J, Gutiérrez-Gutiérrez Ó, Rudolph KL, Thedieck K, Schulze-Späte U, González-Estévez C, Kosan C, Svatoš A, Kwiatkowski M, Koeberle A
Nat Commun 2022, 13(1), 2982 - Identification of dynamic driver sets controlling phenotypical landscapes.
Werle SD, Ikonomi N, Schwab JD, Kraus JM, Weidner FM, Rudolph KL, Pfister AS, Schuler R, Kühl M, Kestler HA
Comput Struct Biotechnol J 2022, 20, 1603-17 - S-nitrosoglutathione reductase (GSNOR) deficiency impairs hematopoietic stem cell proteostasis
Yi W
Dissertation 2022, Jena, Germany
2021
- Nicotinamide for the treatment of heart failure with preserved ejection fraction.
Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, Freundt JK, Voglhuber J, Pricolo MR, Kasa M, Türk C, Aprahamian F, Herrero-Galán E, Hofer SJ, Pendl T, Rech L, Kargl J, Anto-Michel N, Ljubojevic-Holzer S, Schipke J, Brandenberger C, Auer M, Schreiber R, Koyani CN, Heinemann A, Zirlik A, Schmidt A, von Lewinski D, Scherr D, Rainer PP, von Maltzahn J, Mühlfeld C, Krüger M, Frank S, Madeo F, Eisenberg T, Prokesch A, Leite-Moreira AF, Lourenço AP, Alegre-Cebollada J, Kiechl S, Linke WA, Kroemer G, Sedej S
Sci Transl Med 2021, 13(580), eabd7064 - BCL3 couples cancer stem cell enrichment with pancreatic cancer molecular subtypes.
Ai J, Wörmann SM, Görgülü K, Vallespinos M, Zagorac S, Alcala S, Wu N, Kabacaoglu D, Berninger A, Navarro D, Kaya-Aksoy E, Ruess DA, Ciecielski KJ, Kowalska M, Demir EI, Ceyhan GO, Heid I, Braren R, Riemann M, Schreiner S, Hofmann S, Kutschke M, Jastroch M, Slotta-Huspenina J, Muckenhuber A, Schlitter AM, Schmid RM, Steiger K, Diakopoulos KN, Lesina M, Sainz B, Algül H
Gastroenterology 2021, 161(1), 318-32 - Targeting enzyme aging.
Becker F, Rudolph KL
Science 2021, 371(6528), 462-3 - Transient telomere dysfunction induces chromosomal instability and promotes carcinogenesis.
Begus-Nahrmann Y, Hartmann D, Kraus J, Eshraghi P, Scheffold A, Grieb M, Rasche V, Schirmacher P, Lee HW, Kestler HA, Lechel A, Rudolph KL
J Clin Invest 2021, 131(1) Corrigendum for J Clin Invest. 2012;122(6):2283–2288