Subarea 3: Genetics and Epigenetics of Aging
The focus of Subarea 3 is on genetic and epigenetic determinants of life- and health span as well as aging in fish, rodents and humans. This line of research builds on the expertise of the institute in comparative and functional genomics.
The research is defined by five focus areas:
- Comparative genomics in short- and long-lived models of aging,
- Genomic engineering in N. furzeri,
- Epigenetics of aging,
- Non-coding RNAs in aging, and
- Comparative transcriptomics of aging.
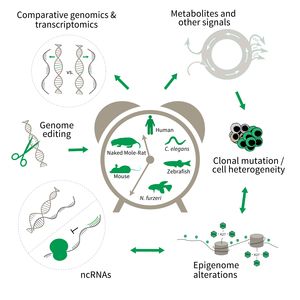
Research focus of Subarea 3.
To uncover causative factors for aging, comparative genomics in short- and long-lived model systems are applied. Functional genomics is used to identify novel pathways contribute to aging of an organism and to validate the functional relevance of genetic and epigenetic changes that occur during aging. Furthermore, genetic risk factors for aging-related diseases are identified and functionally tested. The future development of the Subarea aims to integrate changes in host-microbiota interactions during aging, and how these influence clonal mutation and epigenetic alterations through metabolites and other signals.
Publications
(since 2016)
2024
2023
- Localization and Characterization of Major Neurogenic Niches in the Brain of the Lesser-Spotted Dogfish Scyliorhinus canicula.
Bagnoli S, Chiavacci E, Cellerino A, Terzibasi Tozzini E
Int J Mol Sci 2023, 24(4), 3650 - Distribution of Brain-Derived Neurotrophic Factor in the Brain of the Small-Spotted Catshark Scyliorhinus canicula, and Evolution of Neurotrophins in Basal Vertebrates.
Chiavacci E, Bagnoli S, Cellerino A, Terzibasi Tozzini E
Int J Mol Sci 2023, 24(11), 9495 - Impaired biogenesis of basic proteins impacts multiple hallmarks of the aging brain
Di Fraia* D, Marino* A, Ho Lee* J, Kelmer Sacramento E, Baumgart M, Bagnoli S, Tomaz da Silva P, Kumar Sahu A, Siano G, Tiessen M, Terzibasi-Tozzini E, Gagneur J, Frydman** J, Cellerino** A, Ori** A
bioRxiv 2023, 10.1101/2023.07.20.549210 * equal contribution, ** co-corresponding authors - Establishing the auxin-inducible degron system for Wilms tumor protein 1 degradation in zebrafish
Hopfenmüller V
Dissertation 2023, Jena, Germany - The International Virus Bioinformatics Meeting 2023.
Hufsky F, Abecasis AB, Babaian A, Beck S, Brierley L, Dellicour S, Eggeling C, Elena SF, Gieraths U, Ha AD, Harvey W, Jones TC, Lamkiewicz K, Lovate GL, Lücking D, Machyna M, Nishimura L, Nocke MK, Renard BY, Sakaguchi S, Sakellaridi L, Spangenberg J, Tarradas-Alemany M, Triebel S, Vakulenko Y, Wijesekara RY, González-Candelas F, Krautwurst S, Pérez-Cataluña A, Randazzo W, Sánchez G, Marz M
Viruses 2023, 15(10), 2031 - Sequential disruption of SPLASH-identified vRNA-vRNA interactions challenges their role in influenza A virus genome packaging.
Jakob C, Lovate GL, Desirò D, Gießler L, Smyth RP, Marquet R, Lamkiewicz K, Marz M, Schwemmle M, Bolte H
Nucleic Acids Res 2023, 51(12), 6479 - DNA methylation controls hematopoietic stem cell aging.
Krepelova A, Neri F
Nat Aging 2023, 3(11), 1320-2 - Generation of a transparent killifish line through multiplex CRISPR/Cas9mediated gene inactivation.
Krug J, Perner B, Albertz C, Mörl H, Hopfenmüller VL, Englert C
Elife 2023, 12, e81549 - Rapid Genotyping of Nothobranchius furzeri Embryos, Larvae, and Adult Fish via High-Resolution Melt Analysis (HRMA).
Krug J, Richter A, Englert C
Cold Spring Harb Protoc 2023, 2023(8), 107744