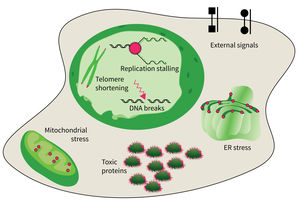
Subarea 4: Cell Dynamics and Molecular Damages in Aging
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2022
- Multifaceted Microcephaly-Related Gene MCPH1
Kristofova M, Ori A, Wang ZQ
Cells 2022, 11(2), 275 - Charakterisierung des potentiellen DNA-Replikationsfaktors PN70
Kutz J
Dissertation 2022, Jena, Germany - Molecular characterization of MAPK10-mediated immune activity and DNA damage responses in hepatocarcinogenesis
Li H
Dissertation 2022, Jena, Germany - Establishment and evaluation of module-based immune-associated gene signature to predict overall survival in patients of colon adenocarcinoma.
Lu J, Annunziata F, Sirvinskas D, Omrani O, Li H, Rasa SMM, Krepelova A, Adam L, Neri F
J Biomed Sci 2022, 29(1), 81 - ADP-ribosyltransferases, an update on function and nomenclature.
Lüscher B, Ahel I, Altmeyer M, Ashworth A, Bai P, Chang P, Cohen M, Corda D, Dantzer F, Daugherty MD, Dawson TM, Dawson VL, Deindl S, Fehr AR, Feijs KLH, Filippov DV, Gagné JP, Grimaldi G, Guettler S, Hoch NC, Hottiger MO, Korn P, Kraus WL, Ladurner A, Lehtiö L, Leung AKL, Lord CJ, Mangerich A, Matic I, Matthews J, Moldovan GL, Moss J, Natoli G, Nielsen ML, Niepel M, Nolte F, Pascal J, Paschal BM, Pawłowski K, Poirier GG, Smith S, Timinszky G, Wang ZQ, Yélamos J, Yu X, Zaja R, Ziegler M
FEBS J 2022, 289(23), 7399-410 - Hanging the coat on a collar: Same function but different localization and mechanism for COPII.
Malis Y, Hirschberg K, Kaether C
Bioessays 2022, 44(10), e2200064 - Meprin β knockout reduces brain Aβ levels and rescues learning and memory impairments in the APP/lon mouse model for Alzheimer's disease.
Marengo L, Armbrust F, Schoenherr C, Storck SE, Schmitt U, Zampar S, Wirths O, Altmeppen H, Glatzel M, Kaether C, Weggen S, Becker-Pauly C, Pietrzik CU
Cell Mol Life Sci 2022, 79(3), 168 - Global metabolic alterations in colorectal cancer cells during irinotecan-induced DNA replication stress.
Marx C, Sonnemann J, Maddocks ODK, Marx-Blümel L, Beyer M, Hoelzer D, Thierbach R, Maletzki C, Linnebacher M, Heinzel T, Krämer OH
Cancer Metab 2022, 10(1), 10 - Meeting Report: Aging Research and Drug Discovery
Meron E, Thaysen M, Angeli S, Antebi A, Barzilai N, Baur JA, Bekker-Jensen S, Birkisdottir M, Bischof E, Bruening J, Brunet A, Buchwalter A, Cabreiro F, Cai S, Chen BH, Ermolaeva M, Ewald Collin Y, Ferrucci L, Florian MC, Fortney K, Freund A, Georgievskaya A, Gladyshev VN, Glass D, Golato T, Gorbunova V, Hoejimakers J, Houtkooper RH, Jager S, Jaksch F, Janssens G, Borch Jensen M, Kaeberlein M, Karsenty G, de Keizer P, Kennedy B, Kirkland JL, Kjaer M, Kroemer G, Lee KF, Lemaitre JM, Liaskos D, Longo VD, Lu YX, MacArthur MR, Maier AB, Manakanatas C, Mitchell SJ, Moskalev A, Niedernhofer L, Ozerov I, Partridge L, Passegué E, Petr MA, Peyer J, Radenkovic D, Rando TA, Rattan S, Riedel CG, Rudolph L, Ai R, Serrano M, Schumacher B, Sinclair DA, Smith R, Suh Y, Taub P, Trapp A, Trendelenburg AU, Valenzano DR, Verburgh K, Verdin E, Vijg J, Westendorp RGJ, Zonari A, Bakula D, Zhavoronkov A, Scheibye-Knudsen M
Aging (Albany NY) 2022, 14(2), 530–543 - A gene dosage-dependent effect unveils NBS1 as both a haploinsufficient tumour suppressor and an essential gene for SHH-medulloblastoma.
Petroni M, Fabretti F, Giulio SD, Robilant VND, Monica VL, Moretti M, Belardinilli F, Bufalieri F, Anna C, Paci P, Corsi A, Smaele ED, Coni S, Canettieri G, Marcotullio LD, Wang ZQ, Giannini G
Neuropathol Appl Neurobiol 2022, 48(6), e12837