Subarea 4: Cell Dynamics and Molecular Damages in Aging
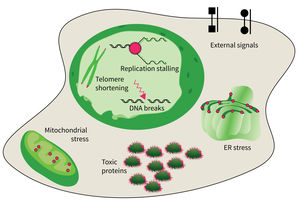
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2025
- DNA damage response and genomic instability disorder: Meeting report of the 20th Ataxia-Telangiectasia Workshop (ATW-2024) in conjunction with the 15th international symposium on DNA damage response and human disease (isDDRHD-2024).
Xia Y, Wang** ZQ, Xu** X
DNA Repair (Amst) 2025 (epub ahead of print) ** co-corresponding authors
2024
- Correction: IER3IP1-mutations cause microcephaly by selective inhibition of ER-Golgi transport.
Anitei M, Bruno F, Valkova C, Dau T, Cirri E, Mestres I, Calegari F, Kaether C
Cell Mol Life Sci 2024, 81(1), 465 - IER3IP1-mutations cause microcephaly by selective inhibition of ER-Golgi transport.
Anitei M, Bruno F, Valkova C, Dau T, Cirri E, Mestres I, Calegari F, Kaether C
Cell Mol Life Sci 2024, 81(1), 334 - Hormesis as an adaptive response to infection.
Bauer M, Ermolaeva M, Singer M, Wetzker R, Soares MP
Trends Mol Med 2024, 30(7), 633-41 - Analysis of the role of YIPF5 in the secretory pathway and microcephaly
Bruno F
Dissertation 2024, Jena, Germany - The Aging Synapse: An Integrated Proteomic And Transcriptomic Analysis
Caterino* C, Ugolini* M, Durso W, Jevdokimenko K, Groth M, Riege K, Görlach M, Fornasiero E, Ori A, Hoffmann S, Cellerino A
bioRxiv 2024, https://doi.org/10.1101/2024.12. * equal contribution - Too old for healthy aging? Exploring age limits of longevity treatments.
Chaudhari PS, Ermolaeva MA
NPJ Metab Health Dis 2024, 2(1), 37 - Amyloid beta precursor protein contributes to brain aging and learning decline in short-lived turquoise killifish (Nothobranchius furzeri)
E.M.de Bakker D, Mihaljević M, Gharat K, Richter Y, Bagnoli S, van Bebber F, Adam L, Shamim-Schulze F, Ohlenschläger O, Bens M, Cirri E, Antebi A, Matić I, Schneider A, Schmid B, Cellerino A, Kirstein J, Riccardo Valenzano D
bioRxiv 2024, https://doi.org/10.1101/2024.10. - Asparagine614 Determines the Transport and Function of the Murine Anti-Aging Protein Klotho.
Fanaei-Kahrani Z, Kaether C
Cells 2024, 13(20), 1743 - PARP1 UFMylation ensures the stability of stalled replication forks.
Gong Y, Wang Z, Zong W, Shi R, Sun W, Wang S, Peng B, Takeda S, Wang ZQ, Xu X
Proc Natl Acad Sci U S A 2024, 121(18), e2322520121