Subarea 4: Cell Dynamics and Molecular Damages in Aging
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
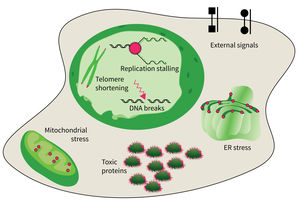
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2023
- The neurological and non-neurological roles of the primary microcephaly-associated protein ASPM.
Wu X, Li Z, Wang ZQ, Xu X
Front Neurosci 2023, 17, 1242448 - The TRRAP-HAT-Sp1 axis maintains brain homeostasis
Yin B
Dissertation 2023, Jena, Germany - TRRAP-mediated acetylation on Sp1 regulates adult neurogenesis.
Yin BK, Lázaro D, Wang ZQ
Comput Struct Biotechnol J 2023, 21, 472
2022
- Deriving life extension strategies from the intestinal microbiome of human centenarians
Chaudhari PS
Dissertation 2022, Jena, Germany - Partial Reduction in BRCA1 Gene Dose Modulates DNA Replication Stress Level and Thereby Contributes to Sensitivity or Resistance.
Classen S, Rahlf E, Jungwirth J, Albers N, Hebestreit LP, Zielinski A, Poole L, Groth M, Koch P, Liehr T, Kankel S, Cordes N, Petersen C, Rothkamm K, Pospiech** H, Borgmann** K
Int J Mol Sci 2022, 23(21), 13363 ** co-corresponding authors - Conserved exchange of paralog proteins during neuronal differentiation.
Di Fraia D, Anitei M, Mackmull MT, Parca L, Behrendt L, Andres-Pons A, Gilmour D, Helmer Citterich M, Kaether C, Beck M, Ori A
Life Sci Alliance 2022, 5(6), e202201397 - The Essential DNA Damage Response Complex MRN Is Dispensable for the Survival and Function of Purkinje Neurons.
Ding* M, Qing* X, Zhang G, Baade-Büttner C, Gruber R, Lu H, Ferguson DO, Geis C, Wang** ZQ, Zhou** ZW
Front Aging Neurosci 2022, 13, 786199 * equal contribution, ** co-senior authors - Career pathways, part 8.
Ermolaeva M, Boyman L
Nat Metab 2022, 4(4), 407-9 - Tight association of autophagy and cell cycle in leukemia cells.
Gschwind* A, Marx* C, Just MD, Severin P, Behring H, Marx-Blümel L, Becker S, Rothenburger L, Förster M, Beck JF, Sonnemann J
Cell Mol Biol Lett 2022, 27(1), 32 * equal contribution - Multifaceted Microcephaly-Related Gene MCPH1
Kristofova M, Ori A, Wang ZQ
Cells 2022, 11(2), 275