Subarea 4: Cell Dynamics and Molecular Damages in Aging
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
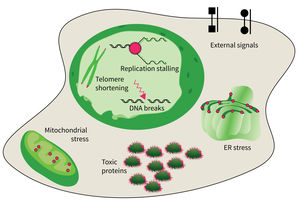
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2021
- Mapping protein carboxymethylation sites provides insights into their role in proteostasis and cell proliferation.
Di Sanzo* S, Spengler* K, Leheis A, Kirkpatrick JM, Rändler TL, Baldensperger T, Dau T, Henning C, Parca L, Marx C, Wang ZQ, Glomb MA, Ori** A, Heller** R
Nat Commun 2021, 12(1), 6743 * equal contribution, ** co-senior authors - Molekularbiologische Beschreibung des Alterungsprozesses
Diekmann S, Grosse F, Hemmerich P, Pospiech H
In: Handbuch Alter und Altern, J.B. Metzler (edited by Fuchs M) 2021, 145–151, Springer, Heidelberg - GMPPA defects cause a neuromuscular disorder with α-dystroglycan hyperglycosylation.
Franzka P, Henze* H, Jung* MJ, Schüler SC, Mittag S, Biskup K, Liebmann L, Kentache T, Morales J, Martínez B, Katona I, Herrmann T, Huebner AK, Hennings JC, Groth S, Gresing LJ, Horstkorte R, Marquardt T, Weis J, Kaether C, Mutchinick OM, Ori A, Huber O, Blanchard V, von Maltzahn J, Hübner CA
J Clin Invest 2021, 131(9), e139076 * equal contribution - Establishment of a fluorescent reporter of RNA-polymerase II activity to identify dormant cells
Freter R, Falletta P, Omrani O, Rasa M, Herbert K, Annunziata F, Minetti A, Krepelova A, Adam L, Käppel S, Rüdiger T, Wang ZQ, Goding** CR, Neri** F
Nat Commun 2021, 12(1), 3318 ** co-corresponding authors - Cell Type-Specific Role of RNA Nuclease SMG6 in Neurogenesis.
Guerra GM, May D, Kroll T, Koch P, Groth M, Wang** ZQ, Li TL, Grigaravičius** P
Cells 2021, 10(12), 3365 ** co-corresponding authors - The function of ATR in post-mitotic neurons and brain homeostasis
Kirtay M
Dissertation 2021, Jena, Germany - ATR regulates neuronal activity by modulating presynaptic firing.
Kirtay M, Sell J, Marx C, Haselmann H, Ceanga M, Zhou ZW, Rahmati V, Kirkpatrick J, Buder K, Grigaravicius P, Ori A, Geis** C, Wang** ZQ
Nat Commun 2021, 12(1), 4067 ** co-corresponding authors - MCPH1, beyond its role deciding the brain size.
Kristofova M, Wang ZQ
Aging (Albany NY) 2021, 13(20), 23437-9 - The Role of the Pathogen Dose and PI3Kγ in Immunometabolic Reprogramming of Microglia for Innate Immune Memory.
Lajqi T, Marx C, Hudalla H, Haas F, Große S, Wang ZQ, Heller R, Bauer M, Wetzker R, Bauer R
Int J Mol Sci 2021, 22(5), 2578 - MAPK10 Expression as a Prognostic Marker of the Immunosuppressive Tumor Microenvironment in Human Hepatocellular Carcinoma.
Li H, Li Y, Zhang Y, Tan B, Huang T, Xiong J, Tan X, Ermolaeva** MA, Fu** L
Front Oncol 2021, 11, 687371 ** co-corresponding authors