Subarea 4: Cell Dynamics and Molecular Damages in Aging
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
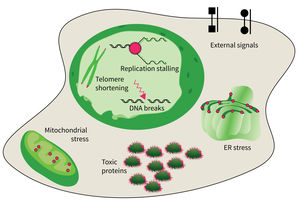
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2022
- Discovering the novel role of the S-adenosyl methionine synthetase SAMS-1 in mediating mitochondrial homeostasis during aging
Poliezhaieva T
Dissertation 2022, Jena, Germany - Circadian clock disruption promotes systemic proteotoxicity via chromatin-mediated gene suppression in C. elegans
Santos Valentim I
Dissertation 2022, Jena, Germany - Oxidative Glucose Metabolism Promotes Senescence in Vascular Endothelial Cells.
Stabenow LK, Zibrova D, Ender C, Helbing DL, Spengler K, Marx C, Wang ZQ, Heller R
Cells 2022, 11(14), 2213 - The Central Domain of MCPH1 Controls Development of the Cerebral Cortex and Gonads in Mice.
Wang Y, Zong W, Sun W, Chen C, Wang** ZQ, Li** T
Cells 2022, 11(17), 2715 ** co-corresponding authors - ASPM promotes ATR-CHK1 activation and stabilizes stalled replication forks in response to replication stress.
Wu* X, Xu* S, Wang P, Wang ZQ, Chen H, Xu X, Peng B
Proc Natl Acad Sci U S A 2022, 119(40), e2203783119 * equal contribution - PARP1: Liaison of Chromatin Remodeling and Transcription.
Zong W, Gong Y, Sun W, Li T, Wang ZQ
Cancers (Basel) 2022, 14(17), 4162
2021
- The role of Atlastin-3 in hereditary axonopathies / by B.Sc., M.Sc., Laura Behrendt
Behrendt L
Dissertation 2021, Jena, Germany - Disease-causing mutated ATLASTIN 3 is excluded from distal axons and reduces axonal autophagy.
Behrendt L, Hoischen C, Kaether C
Neurobiol Dis 2021, 155, 105400 - Nbs1-mediated DNA damage repair pathway regulates haematopoietic stem cell development and embryonic haematopoiesis.
Chen Y, Sun J, Ju Z, Wang ZQ, Li T
Cell Prolif 2021, 54(3), e12972 - Mitochondrial dysfunction and loss of metabolic plasticity cause a reversal of metformin longevity benefits in late life
Dakhovnik O
Dissertation 2021, Jena, Germany