To better understand the interaction between the process of aging and the diseases of aging, researchers from the Jena Centre for Systems Biology of Ageing (JenAge) performed the most comprehensive comparison of aging across species and tissues that has been undertaken to date. “Comparative systems-wide approaches are powerful tools to dissect complex biomedical processes like aging,” says JenAge coordinator Dr Jürgen Sühnel from the Leibniz Institute on Aging – Fritz Lipmann Institute, Jena. The present analysis was coordinated by Professor Christoph Kaleta, now a member of the Cluster of Excellence “Inflammation at Interfaces”, from Kiel University in Germany and Professor Eytan Ruppin, University of Maryland, USA. The scientists compared changes in the activity of genes during aging from humans, mice, the zebrafish Danio rerio and the short-lived killifish Nothobranchius furzeri across several organs. “We found many similarities in the molecular signatures of age-associated changes in the investigated species. Our results clearly indicate that the core of aging is highly similar among very different vertebrates, whose natural lifespans range from a few months to almost a century,” says Professor Kaleta, the corresponding author of the study.
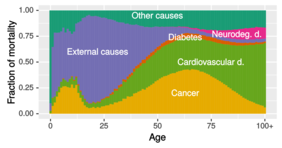
Subsequently, the researchers compared the changes in gene activity with the signatures of age-specific diseases. Age-related changes in gene activity were accompanied by changes observed in degenerative diseases. Surprisingly, those changes showed a reverse development compared to cancer. “Our results indicate that aging does not necessarily promote all age-related diseases, but might have very disease-specific effects,” elaborates Professor Kaleta. Indeed, the different influence of aging on diseases might provide an explanation as to why, at an advanced age, the likelihood of dying of cancer is decreasing compared to degenerative diseases. Intriguingly, the researchers found this difference between cancer and degenerative diseases is also visible on the genomic level: many risk genes that increase the chance of getting a degenerative disease actually protect against cancer and vice versa.
While the researchers can only speculate about the cause for the opposite direction of changes in gene activity between cancer and degenerative diseases, they point out that it might be related to the continuous accumulation of damaged DNA (deoxyribonucleic acid) in cells when humans get older. This raises the risk of getting cancer. As a consequence, the immune system is activated to destroy or keep the damaged cells in check to prevent cancer from developing. A chronic low grade inflammation, which is often observed in the elderly, might be a consequence of the latter, facilitating further degenerative tissue damage. “As the molecular signatures of cancer and degenerative disorders oppose each other in key cellular processes, it logically follows that the molecular alterations occurring late in life may thus not be able to drive away both, cancer and degenerative disorders, at the same time,” says Professor Ruppin from the University of Maryland. “Indeed, our results show that they are aligned to counteract the increased risk of cancer but, probably inevitably, the price might be an increased risk of a degenerative disease.”
The new data clearly indicates a strong connection between degenerative diseases and cancer. These results are also of particular importance regarding the current search for treatments that reverse age-associated changes. In light of the new results such approaches might have to be carefully evaluated with regard to their effect on cancer development.
Original publication
Aramillo Irizar, P, Schäuble, S, Esser, D, Groth, M, Frahm, C, Priebe, S, Baumgart, M, Hartmann, N, Marthandan, S, Menzel, U, Müller, J, Schmidt, S, Ast, V, Caliebe, A, König, R, Krawczak, M, Ristow, M, Schuster, S, Cellerino, A, Diekmann, S, Englert, C, Hemmerich, P, Sühnel, J, Guthke, R, Witte, OW, Platzer, M, Ruppin, E and Kaleta, C (2018): Transcriptomic alterations during ageing reflect the shift from cancer to degenerative diseases in the elderly. Nature Communications, dx.doi.org/10.1038/s41467-017-02395-2
Contact
Professor Christoph Kaleta Cluster of Excellence “Inflammation at Interfaces” and Institute of Experimental Medicine, Kiel University E-mail: c.kaleta@iem.uni-kiel.de Tel.: +49 (0)431 500-30340