Jena/Cambridge. The recycling program of cells is autophagy; a fundamental component of the cellular quality control and extremely important for maintaining cell functionality. Degradation of misfolded proteins or damaged organelles through autophagy prevents negative effects on the cell and allows nutrients recycling so they can be used by the cell to build up new components. Errors in this recycling process can lead to cancer, neurodegenerative diseases and increased susceptibility to infections, and they can also affect ageing.
Under normal conditions, autophagy takes place only to a small extent; however it is activated under nutrient starvation, e.g. deficiency of amino acids. It is known, that the “mechanistic target of rapamycin complex 1” (mTORC1), the major nutrient-sensitive regulator of growth, plays an important role in this process. It regulates the flow of macromolecules intended for degradation to the "stomach of the cell", the lysosomes. How this intracellular regulation works under starvation and what proteins play a role in it, are still open questions.
Insights into the recycling process of the cell
Researchers from the lab of Alessandro Ori at the Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) followed these questions in cooperation with the lab of David M. Sabatini from the Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology (MIT), Cambridge, Massachusetts, USA. They performed quantitative proteomic analyses to analyze the lysosomal proteome under conditions that inhibit the mTORC1 pathway. They examined lysosomes in nutrient-replete media (full media), under conditions of starvation, through deficiency of amino acids and glucose, and under conditions that inhibit mTOR kinase through torin1.
“Thanks to the LysoIP method that was developed in the Sabatini Lab and the advanced mass spectrometry platform of the FLI core facility proteomics, we were able to profile the dynamics of the lysosomal proteome under different conditions of mTORC1 activation”, tells Dr. Alessandro Ori, Junior Group Leader at the FLI. The international team identified more than 800 proteins associated with lysosomes. “Of these many proteins, the Nuclear fragile X mental retardation-interacting protein 1 (NUFIP1) piqued our interest, because its lysosomal abundance increased upon the inhibition of mTORC1 pathway.”
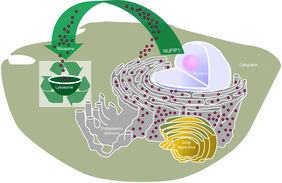
Migration of NUFIP1 upon mTOR inhibition
Previous work indicates that NUFIP1 is largely a nuclear protein but it has also been observed in the cytoplasm of some cell types. With biochemical assays and imaging studies the collaborators from MIT were able to confirm that the inhibition of mTOR caused NUFIP1 to translocate from the nucleus to autophagosomes and lysosomes. “These are both cell components that play an important role in autophagy”, underlines Dr. Ori.
NUFIP1 is important to survive starvation
With these “snapshots into the cells” the researchers were able to identify this new role of the protein NUFIP1, as it was previously unknown that it is also associated with lysosomes. Upon starvation NUFIP1 binds intact ribosomes and targets them for degradation in the lysosomes. Ribosomes, the “protein factory” of cells, are large complexes made up of proteins and RNAs. They are among the most abundant components of cells and, as such, they represent one of the most important reserves of nutrients. “We were now able to show that NUFIP1 is necessary for ribosomal degradation upon starvation in human and mouse cells and with that ensures the survival of the cell in hunger situations", emphasizes Dr. Ori.
The results of the research team from Germany and the USA underline that the protein NUFIP1 plays an important role in the selective autophagy of ribosomes in cases of nutrient starvation in mammalian cells. This work exemplifies how the study of the dynamics of organelle proteomes can reveal fundamental mechanisms that are relevant for biomedical and aging research.
Publication
NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Gregory A. Wyant, Monther Abu-Remaileh, Evgeni M. Frenkel, Nouf N. Laqtom, Vimisha Dharamdasani, Caroline A. Lewis, Sze Ham Chan, Ivonne Heinze, Alessandro Ori, David M. Sabatini. Science 2018, eaar2663, DOI: 10.1126/science.aar2663. science.sciencemag.org/content/early/2018/04/25/science.aar2663
Contact
Dr. Kerstin Wagner
Press and Public Relations
Phone: 03641-656378
Email: presse@leibniz-fli.de