Subarea 5: Computational and Systems Biology of Aging
Subarea 5 focuses on the development of methods to analyse and understand complex biological systems. This work includes the design of computer algorithms and biostatistical approaches as well as the development of novel Omic strategies (i.e. genomics/epigenomics, transcriptomics, proteomics, and metabolomics) to study aging and aging-related diseases. According to the FLI, due to the Subarea's expertise in computational data analysis, it is deeply interconnected with all other Subareas. The Subarea hosts two critical core facilities (Life Science Computing, Proteomics) and provides consulting services in statistics. Furthermore, it organizes courses on data analysis and statistics.
The research is defined by five focus areas:
- Mapping extrinsic and intrinsic factors influencing stem cells during aging,
- Integration of spatiotemporal proteomics and transcriptomics data,
- Comprehensive evaluation of qualitative and quantitative expression changes,
- Identification and analysis of epigenomic alterations during aging and age-related diseases, and
- Network analysis of genomic, transcriptomic and epigenomic alterations during aging.
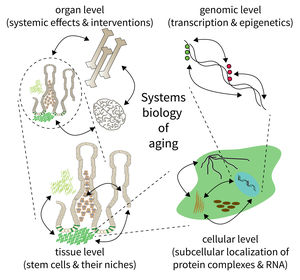
Research focus of Subarea 5.
The biology of aging can be viewed as a multilayered array of networks at the level of organs, cells, molecules, and genes. The FLI wants to meet this complexity by establishing the new Subarea on “Computational and Systems Biology of Aging”. The overall goal is to interconnect research at different scales, taking place in Subareas 1-4 of the Institute’s research program. The new group on Systems Biology will integrate data from networks at multiple scales and will thus point to mechanisms and interactions that would not be seen in unilayer approaches.
Publications
(since 2016)
2023
- Author Correction: Patterns of somatic structural variation in human cancer genomes.
Li Y, Roberts ND, Wala JA, Shapira O, Schumacher SE, Kumar K, Khurana E, Waszak S, Korbel JO, Haber JE, Imielinski M, PCAWG Structural Variation Working Group, Weischenfeldt J, Beroukhim R, Campbell PJ, PCAWG Consortium
Nature 2023, 614(7948), E38 - Focal structural variants revealed by whole genome sequencing disrupt the histone demethylase KDM4C in B cell lymphomas.
Lopez C, Schleussner N, Bernhart SH, Kleinheinz K, Sungalee S, Sczakiel HL, Kretzmer H, Toprak UH, Glaser S, Wagener R, Ammerpohl O, Bens S, Giefing M, Sanchez JCG, Apic G, Hubschmann D, Janz M, Kreuz M, Mottok A, Muller JM, Seufert J, Hoffmann S, Korbel JO, Russell RB, Schule R, Trumper L, Klapper W, Radlwimmer B, Lichter P, Kuppers R, Schlesner M, Mathas S, Siebert R
Haematologica 2023, 108(2), 543-54 - Remodeling of the protein ubiquitylation landscape in the aging vertebrate brain
Marino* A, Di Fraia* D, Panfilova D, Kumar Sahu A, Ori A
bioRxiv 2023, 10.1101/2023.12.02.569713 * equal contribution - Age-Associated Changes in Endothelial Transcriptome and Epigenetic Landscapes Correlate With Elevated Risk of Cerebral Microbleeds.
Mohan K, Gasparoni G, Salhab A, Orlich MM, Geffers R, Hoffmann S, Adams RH, Walter J, Nordheim A
J Am Heart Assoc 2023, 12(17), e031044 - Spontaneous onset of cellular markers of inflammation and genome instability during aging in the immune niche of the naturally short-lived turquoise killifish (Nothobranchius furzeri)
Morabito G, Dönertas HM, Seidel J, Poursadegh A, Poeschla M, Valenzano DR
bioRxiv 2023 - The HLA ligandome of oropharyngeal squamous cell carcinomas reveals shared tumour-exclusive peptides for semi-personalised vaccination.
Mühlenbruch L, Abou-Kors T, Dubbelaar ML, Bichmann L, Kohlbacher O, Bens M, Thomas J, Ezić J, Kraus JM, Kestler HA, von Witzleben A, Mytilineos J, Fürst D, Engelhardt D, Doescher J, Greve J, Schuler PJ, Theodoraki MN, Brunner C, Hoffmann TK, Rammensee HG, Walz JS, Laban S
Br J Cancer 2023, 128(9), 1777-87 - Performance of qpAdm-based screens for genetic admixture on admixture-graph-shaped histories and stepping-stone landscapes
Olga F, Ulaş I, Eren Y, Matthew PW, Christian DH, Jan K, Leonid AV, Piya C, Pavel F
bioRxiv 2023, https://doi.org/10.1101/2023.04. - Author Correction: IFNγ-Stat1 axis drives aging-associated loss of intestinal tissue homeostasis and regeneration.
Omrani* O, Krepelova* A, Rasa SMM, Sirvinskas D, Lu J, Annunziata F, Garside G, Bajwa S, Reinhardt S, Adam L, Käppel S, Ducano N, Donna D, Ori A, Oliviero S, Rudolph KL, Neri F
Nat Commun 2023, 14(1), 6302 * equal contribution - IFNγ-Stat1 axis drives aging-associated loss of intestinal tissue homeostasis and regeneration.
Omrani* O, Krepelova* A, Rasa SMM, Sirvinskas D, Lu J, Annunziata F, Garside G, Bajwa S, Reinhardt S, Adam L, Käppel S, Ducano N, Donna D, Ori A, Oliviero S, Rudolph KL, Neri F
Nat Commun 2023, 14(1), 6109 * equal contribution - Author Correction: Genomic basis for RNA alterations in cancer.
PCAWG Transcriptome Core Group, Calabrese C, Davidson NR, Demircioğlu D, Fonseca NA, He Y, Kahles A, Lehmann KV, Liu F, Shiraishi Y, Soulette CM, Urban L, Greger L, Li S, Liu D, Perry MD, Xiang Q, Zhang F, Zhang J, Bailey P, Erkek S, Hoadley KA, Hou Y, Huska MR, Kilpinen H, Korbel JO, Marin MG, Markowski J, Nandi T, Pan-Hammarström Q, Pedamallu CS, Siebert R, Stark SG, Su H, Tan P, Waszak SM, Yung C, Zhu S, Awadalla P, Creighton CJ, Meyerson M, Ouellette BFF, Wu K, Yang H, PCAWG Transcriptome Working Group, Brazma A, Brooks AN, Göke J, Rätsch G, Schwarz RF, Stegle O, Zhang Z, PCAWG Consortium
Nature 2023, 614(7948), E37